
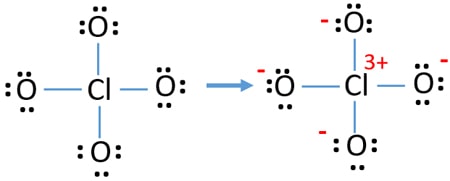

#PERCHLORATE ION BONDING PAIRS SERIES#
Selective uptake of cationic organic dyes in a series of isostructural Co2+/Cd2+ metal-doped metal–organic frameworks. Shuang Chen, Cui-juan Wang, Dong-ning Liu, Zi-xin Zhu, Yan-yu Qian, Dan Luo, Yao-yu Wang.Symmetric quaternary phosphonium cation and perchlorate/chlorate anions: Crystal structure, Database study and Hirshfeld surface analysis. Fe6 clusters of tripodal alcohol ligands: Synthesis, structures and magnetostructural properties. Mo Ashafaq, Mohd Khalid, Mukul Raizada, Anzar Ali, Mohd Faizan, M.) dithiolate coordination polymers: crystallographic, computational, Hirshfeld surface and thermal analyses. Pooja Singh, Amita Singh, Ayushi Singh, Ashish Kumar Singh, Gabriele Kociok-Köhn, Ahmad Alowais, Naaser A.Monatshefte für Chemie - Chemical Monthly 2020, 151 Synthesis, single-crystal X-ray diffraction, and in vitro biological evaluation of sodium, cobalt, and tin complexes of o-nitro-/o-methoxyphenylacetic acid: experimental and theoretical investigation. Muhammad Danish, Muhammad Asam Raza, Sana Iftikhar, Muhammad Waseem Mumtaz, Muhammad Nawaz Tahir, Umer Rashid, Khurshid Ayub.Supramolecular assemblies involving salt bridges: DFT and X-ray evidence of bipolarity. Suparna Tripathi, Samiul Islam, Saikat Kumar Seth, Antonio Bauzá, Antonio Frontera, Subrata Mukhopadhyay.Anion-dependent structural variations and charge transport property analysis of 4′-(3-pyridyl)-4,2′:6′,4′′-terpyridinium salts. Anowar Hossain, Arka Dey, Saikat Kumar Seth, Partha Pratim Ray, Joaquín Ortega-Castro, Antonio Frontera, Subrata Mukhopadhyay.Synthesis, spectral property, IEF-PCM solvation, anti-microbial evaluation and molecular docking studies of 6 amino-2-(4 nitrophenyl)-1H-benzimidazole. The Journal of Physical Chemistry A 2013, 117 ♼l Interactions in Molecular Crystals: Insights from the Theoretical Charge Density Analysis.♼lO4– Interactions and the Interplay between π+–π and Anion.Experimental and Computational Study of Counterintuitive ClO4– Prankrishna Manna, Saikat Kumar Seth, Monojit Mitra, Somnath Ray Choudhury, Antonio Bauzá, Antonio Frontera, and Subrata Mukhopadhyay.The Journal of Physical Chemistry B 2014, 118 3-Picoline Mediated Self-Assembly of M(II)–Malonate Complexes (M = Ni/Co/Mn/Mg/Zn/Cu) Assisted by Various Weak Forces Involving Lone Pair−π, π–π, and Anion Monojit Mitra, Prankrishna Manna, Antonio Bauzá, Pablo Ballester, Saikat Kumar Seth, Somnath Ray Choudhury, Antonio Frontera, and Subrata Mukhopadhyay.Chameleon-like Nature of Anagostic Interactions and Its Impact on Metalloaromaticity in Square-Planar Nickel Complexes. Babashkina, Koen Robeyns, Filip Sagan, Dariusz W. Influence of 2-Amino-4-methylpyridine and 2-Aminopyrimidine Ligands on the Malonic Acid-Cu(II) System: Insights through Supramolecular Interactions and Photoresponse Properties. Rheingold, Partha Pratim Ray, Antonio Frontera, Subrata Mukhopadhyay. Tripti Mandal, Arka Dey, Saikat Kumar Seth, Joaquín Ortega-Castro, Arnold L.This article is cited by 27 publications. We describe the energetic and geometric features of this lone pair–salt-bridge interaction and explore its impact on the resultant supramolecular organization using theoretical DFT-D3 calculations. The combination of this interaction with various other weak intermolecular forces results in a remarkably extended supramolecular network combining a wide variety of interactions involving π–systems (Cl
π interactions and an unexplored interaction between the lone pair (lp) of one malonate oxygen atom and a planar salt bridge.Examining this characteristic of the solid-state architecture, we noticed several salt-bridge (sb) A multitude of salt bridges are formed in this structure, connecting the protonated 2-amino-5-chloropyridines and the malonate ligands of the polymeric polyanion. These 2D sheets are joined side by side primarily by various hydrogen bonds to form a 3D structure. In 1, copper(II) malonate units form infinite 1D polymeric chains, which are interlinked by hydrogen bonds to generate 2D sheets. A Cu(II)–malonate complex with formula n ( 1) has been synthesized from purely aqueous media simple by mixing the reactants in their stoichiometric ratio, and its crystal structure has been determined by single-crystal X-ray diffraction.


 0 kommentar(er)
0 kommentar(er)
